When you’d like to grasp more details on Fluke Biomedical Exam Gear, our solution industry experts are below that can help. Complete the shape and somebody offers you a contact to answer your concerns.
It’s advised to run just one unfavorable Manage for TSB and just one destructive Manage for FTM each check working day for each microbiologist executing testing.
Staying proactive assists produce a collaborative romantic relationship and ensures that the protocol aligns with their expectations.
The elements must be effectively sterilized working with suited sterilization strategies that won't have an affect on the standard, texture as well as the Organic exercise inherent in them. Raw materials that are not sterilized and those that are for being processed even more just after sterilization needs to be handled aseptically to avoid possible contamination possibly for the duration of storage or dealing with.
Sterility indicators are made use of to check the standard and checking of sterilization procedures. They can show no matter whether microbial growth happens or sterilization was helpful. There are various sorts of sterility indicators for various sterilization methods including dry heat, moist heat, gaseous, radiation, and filtration sterilization.
Sample template on how to publish your exploration achievements and final results when applying for just a fellowship or grant
Additionally, as pointed out, each terminal sterilized sublot of a product batch really should be tested separately based on the regulatory specifications outlined in USP 71. The amount of vials examined is set by the dimensions of each sublot.
See what our attendees considered this year's party and maintain an eye fixed out for more aspects on our 2024 conference.
But how often really should corporations perform these exams to strike the best stability in between effectiveness and usefulness?
Completely ready-to-use Good quality Manage Strategies aid the full choice of biosafety testing at each individual phase, shortening the time it takes to obtain outcomes.
Surgical instruments employed in operations must be free of charge from microbes as a way to stop postoperative infections.
I would like to sign up for newsletters from Sartorius (Sartorius AG and its affiliated firms) primarily based of my private pursuits.
Frequently, sterility testing is usually a regulatory prerequisite for the release of biological and pharmaceutical products that can not be terminally sterilized (i.e. products which can be warmth-labile and so liable to destruction by warmth); and sterility examination even now continue being a move for the release of Organic products for public usage. Since sterility testing simply cannot on its own certify absolutely the assurance of freedom of a product from microbial contamination, it is significant that every production procedures (Particularly those intended for the creation of biological products) guarantees a continued and rigorous compliance to Excellent Producing Practices (GMPs) at every single here production stage.
2. Sluggish Advancement or No Growth: Some microorganisms might have gradual expansion prices or may well not mature beneath the incubation circumstances Utilized in sterility testing. This may lead to Bogus-damaging effects. Validation scientific tests really should be conducted to make sure the appropriateness from the picked incubation ailments.
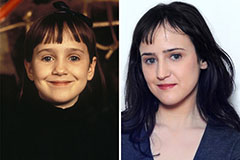 Mara Wilson Then & Now!
Mara Wilson Then & Now! Angus T. Jones Then & Now!
Angus T. Jones Then & Now! Lark Voorhies Then & Now!
Lark Voorhies Then & Now! Monica Lewinsky Then & Now!
Monica Lewinsky Then & Now! Daryl Hannah Then & Now!
Daryl Hannah Then & Now!